Research Activities (brief)
Utilizing theoretical methods, such as quantum chemical calculations and
molecular dynamics simulations, we are working on various aspects of
liquid systems, biomolecules, and other condensed phase systems,
particularly on their structural, dynamical, spectroscopic, and
functional properties as well as the intermolecular interactions
involved therein.
Interactions between Proteins and Solvents and/or Ligands
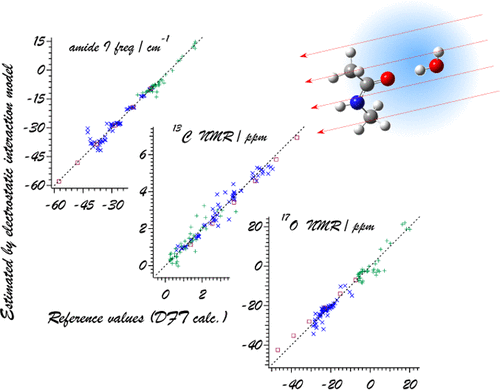
The intermolecular interactions between proteins and solvent water molecules
play an important role in their structural formations and functions. It is
also known that some halogenated ligands interact with proteins by halogen bonding.
In the vibrational (IR and Raman) spectra of proteins, there are some bands
that provide us information on the structural and dynamical properties and
the interactions with solvents and ligands. Electrostatic interactions and
the behavior of electrons play especially important roles. We are conducting
theoretical analyses on these points.
・
J. Phys. Chem. B 122, 154-164 (2018).
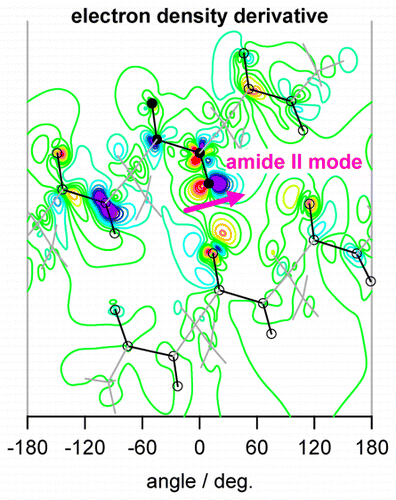
・
J. Phys. Chem. B 120, 1624-1634 (2016).
Behavior of Electrons and the Terahertz Spectral Intensities
of Hydrogen-Bonding Systems
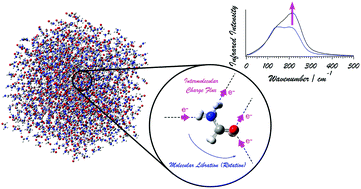
Terahertz Spectroscopy is a promising powerful method for measuring the
structures and dynamics of hydrogen-bonding systems. It is becoming
clear that the behavior of electrons is intimately related to the
spectral intensities. We are conducting theoretical analyses on this point,
as well as the intensity generation mechanisms of the Raman spectra
in the same low-frequency region.
・
Phys. Chem. Chem. Phys. 20, 3029-3039 (2018).
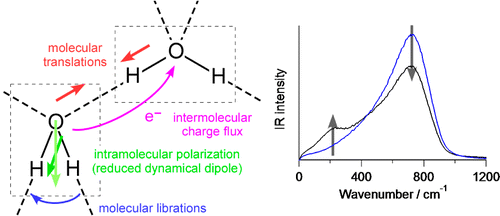
・
J. Chem. Theory Comput. 10, 1219-1227 (2014).
Structural Formation around Ions in Electrolyte Solutions
One important subject in relation to the properties of electrolyte solutions
is the structural formation around the ions.
Especially in mixed-solvent systems, there are some cases where preferential
solvation is taking place, contributing to the functional properties of the
solutions. Shown below is an example where the preferential solvation in the
solution of LiClO4 in propylene carbonate and diethyl carbonate
was elucidated by Raman spectroscopy and theoretical analysis.
Metal ions are also contained in some proteins, contributing to their
functions in various ways. We are conducting theoretical analysis also on this point.
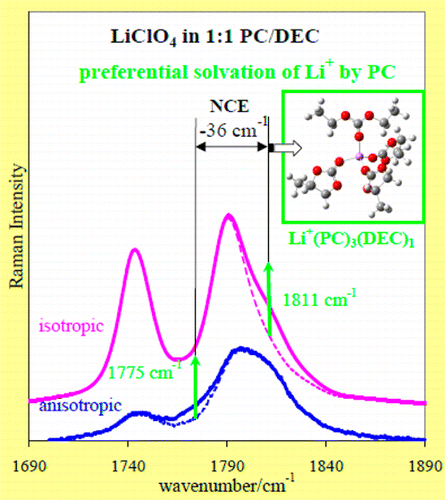
・M. G. Giorgini, K. Futamatagawa, H. Torii, M. Musso, and S. Cerini,
J. Phys. Chem. Lett. 6, 3296-3302 (2015).
Related Basic Theories
In relation to the subjects explained above, various studies on the related
basic theories are also conducted.
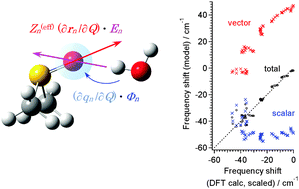
・
Phys. Chem. Chem. Phys. 18, 10081-10096 (2016).
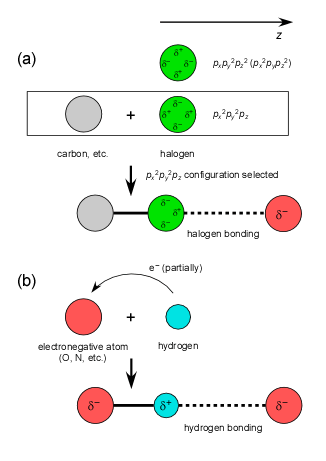
・
J. Comput. Chem. 31, 107-116 (2010).
・
AIP Conference Proceedings 1504, 228-239 (2012).






